Overcoming the Challenge of Highly Polar Impurity Analysis in Drug Products
When Standard Methods Fail: A Critical Analytical Challenge
Pharmaceutical companies face increasing pressure to detect and quantify impurities that can impact drug product stability and patient safety. When these impurities are highly polar compounds, they present unique analytical challenges that standard HPLC methods often cannot resolve.The Problem:
An Impurity Hidden in Plain Sight
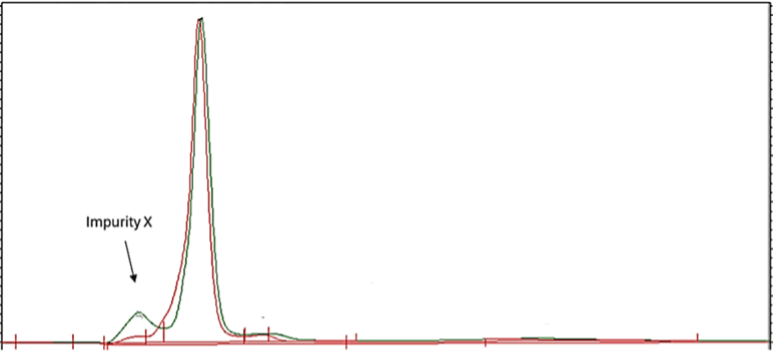
A company’s analytical team identified an impurity growing in their drug product: Impurity X was flagged as a potential genotoxic impurity which was growing on stability in their drug product.
This wasn’t just a minor issue, since impurity growth posed a high risk to the product release and regulatory approval.
The challenge was intensified by the impurity’s chemical nature:
- Impurity X exhibited extreme polarity
- Poor retention on the HPLC column caused it to elute at the solvent front
- The existing analytical method couldn’t distinguish it from other early-eluting compounds
- Co-elution with other components prevented accurate quantification
- Without resolution and quantification, it was impossible to determine if impurity levels were within acceptable limits of the specification
The risks were high: inadequate analytical methods mean delayed product release, potential regulatory setbacks, and ultimately, delayed patient access to essential medications.

THE RESOLIAN APPROACH:
EXPERT METHOD DEVELOPMENT
The client needed our analytical expertise to overhaul and redevelop the insufficient method into a robust and validated solution.
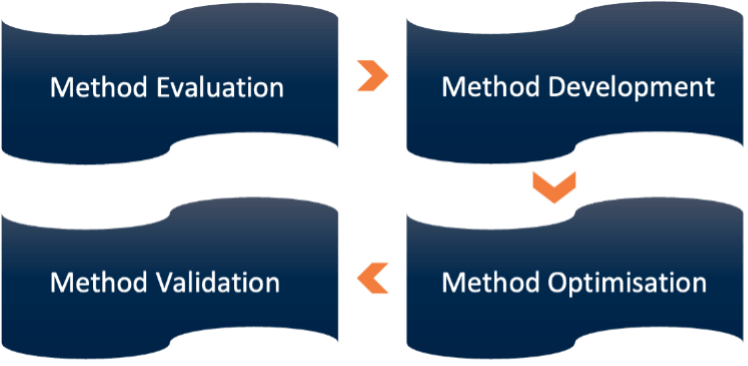
Resolian’s analytical science team approached the challenge systematically, applying extensive knowledge of chromatographic principles and method development for challenging polar compounds:
Method Development Phase
Our scientists explored multiple chromatographic variables to achieve retention and separation of highly polar compounds:
- Column chemistry optimisation
- Mobile phase composition adjustments
- pH and buffer system evaluation
- Gradient profile refinement
Method Optimisation Phase
Once initial separation was achieved, the method underwent rigorous optimisation to ensure:
- Baseline resolution of all critical components
- Peak purity confirmation
- MS compatibility for structural confirmation
- Method Robustness
Method Validation Phase
The optimised method was validated to GMP standards, ensuring it met all regulatory requirements for pharmaceutical quality control.
THE RESULTS:
From Inadequate to Compliant
The new validated method addressed all the customer concerns.
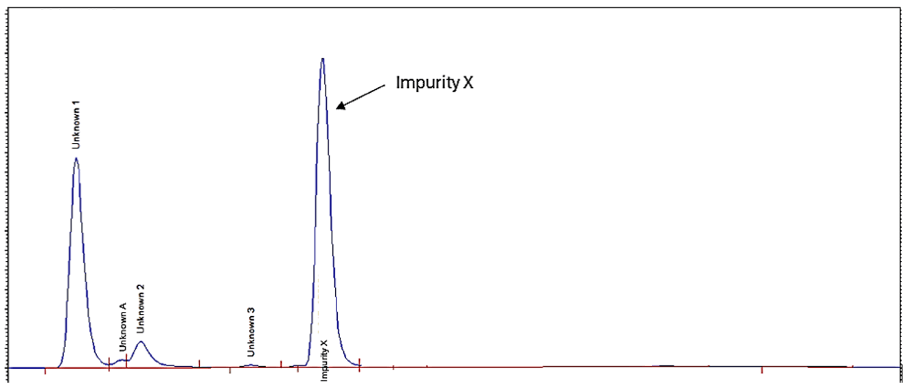
Where the original method provided incomplete information, the Resolian method delivered the complete quantification of Impurity X:
| Parameter | Original Method | Resolian Method |
| Impurity X Resolution | Not resolved | Baseline resolved |
| Impurities Separated | 2 (including X) | 5 (including X) |
| Impurity X Retention Time | Eluting at solvent front | Well Resolved from the solvent front |
| Peak Purity | Not pure (co-elution) | Pure |
| MS Compatibility | Yes | Yes |
The impact of the new method design provided:
- Regulatory confidence:
A validated, robust method that meets GMP requirements - Stability program success:
Accurate trending of impurity growth over time - Product release capability:
Clear, quantifiable impurity data for quality decision-making - Extended method ability:
A method that separates not just Impurity X, but other components previously undetected that could pose future challenges
Why Polar Compounds Require Specialised Expertise
Highly polar impurities represent one of the most challenging areas of pharmaceutical analysis.
These molecules:
- Exhibit weak interaction with traditional reversed-phase columns
- Often require specialised stationary phases or mobile phase conditions
- Can co-elute with solvent fronts, masking critical quality attributes
- May require unique detection strategies for accurate quantification
Developing methods for these compounds isn’t simply about following a protocol – it requires deep understanding of chromatographic theory, extensive experience with challenging separations, and the analytical creativity to find solutions when standard approaches fail.
Your Analytical Challenges Deserve Expert Solutions
Is your team struggling with:
- Polar impurities that won’t retain on your column?
- Methods that worked in development but fail during stability studies?
- Impurities retained in the solvent front or co-eluting with other components?
- Regulatory concerns about your analytical methods?
Resolian’s analytical science team specialises in solving complex separation challenges. From Drug Products to other difficult-to-analyse molecules, we bring the expertise to develop, optimise, and validate methods that deliver the analytical confidence you need.
Our Analytical Scientific Services include:
- Advanced Method Development (GMP method development and validation)
- Complex Sample Preparation Expertise
- Forced Degradation Studies
- Regulatory-Ready Method Validation
- Method Transfer & Feasibility Studies
- Analytical Troubleshooting & Redevelopment
- Routine Testing & Physicochemical Analysis
- Complex separation problem-solving
- Impurity identification and quantification
- High resolution MS and structural elucidation
- Stability-indicating method development
- MS-compatible method design
- Regulatory-compliant analytical packages

Ready to Solve Your Analytical Challenges?
Don’t let inadequate analytical methods compromise your product development timeline or regulatory strategy.
Contact Resolian today to discuss how our analytical sciences team can provide the expertise and solutions you need.