Turning Complexity into Clarity: Resolving siRNA Bioanalysis Challenges: How Resolian helps pharma and biotech partners overcome LC-MS hurdles for oligonucleotide quantitation.
Small interfering RNA (siRNA) therapies are transforming the treatment landscape for genetic diseases – but their promise comes with analytical complexity. High polarity, instability, and low plasma concentrations make accurate quantitation a formidable challenge for sponsors.
In this article, we share how Resolian partnered with a leading pharma innovator to tackle these obstacles head-on. From mitigating lipemic matrix effects to achieving reliable selectivity, our scientists applied a multi-stage LC-MS strategy that delivered a fully validated method meeting ICH M10 standards.
This case study demonstrates more than technical expertise – it shows how collaboration and innovation can accelerate the development of next-generation therapeutics.
The Background
Small interfering RNA (siRNA) therapeutics are revolutionizing the treatment of genetic diseases through targeted gene silencing. However, their size, polarity, and instability make bioanalysis particularly challenging. Sponsors developing siRNA drugs require robust LC-MS methods that can quantify low plasma levels accurately and meet regulatory validation standards.The Challenges
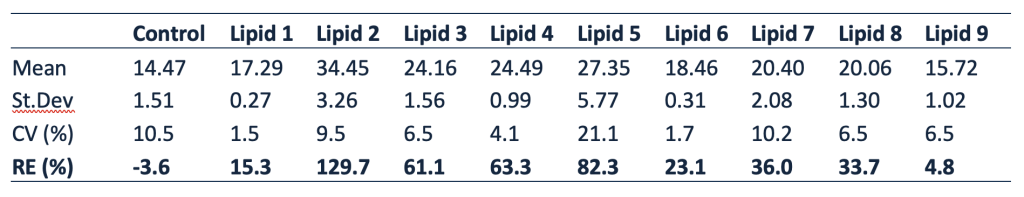
Resolian scientists faced several complex analytical hurdles:- Matrix Effects: Lipemic plasma introduced significant positive bias (>+15% RE in 8 of 9 matrices).
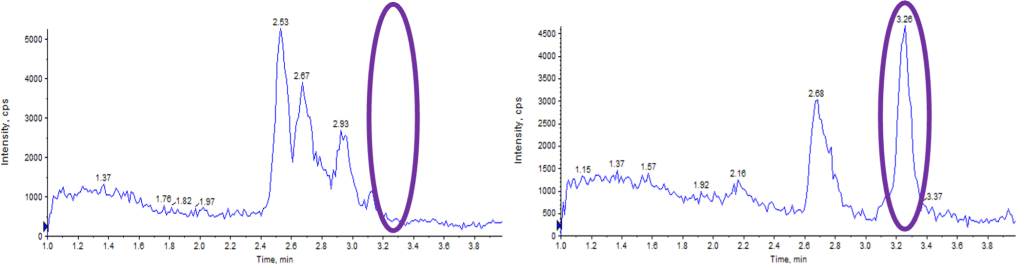
- Selectivity Issues: Co-eluting interferences and cross-talk from n-1 metabolites risked inaccurate parent drug quantitation.
The Solution
A multi-stage approach was applied to overcome these challenges:- Sample Preparation: Implemented weak anion-exchange SPE with optimized wash steps, including acidic and non-polar washes.
- Chromatographic Optimization: Screened columns and mobile phases, adopting a 300ÅBEH C18 column with HFIP/DBA additives to achieve separation of parent drug from n-1 metabolite.
- Matrix Effect Resolution: Diluted extracts 1:5 and adapted wash steps to remove lipemic interference, resulting in normalized matrix factors within acceptable range.
The Results
- Validated Method: Fully validated per ICH M10 guidelines for ng/mL quantitation.
- Improved Accuracy: Lipemic matrix RE reduced to <±15%, meeting regulatory expectations.
- Reliable Selectivity: Achieved chromatographic separation of parent drug and n-1 metabolite, enabling accurate clinical sample analysis.
Ready to resolve your challenges?
Resolian partners with leading pharma and biotech companies to solve complex oligonucleotide and peptide bioanalysis challenges. Learn more about our bioanalytical solutions

