Orally Inhaled and Nasal Drug Products (OINDPs) such as pressurized metered-dose inhalers (pMDIs), dry powder inhalers (DPIs),
and nebulized formulations are engineered to deliver precise doses directly to the respiratory tract.
Any foreign particulate matter (FPM) present in these products can compromise patient safety, product performance,
and regulatory compliance.

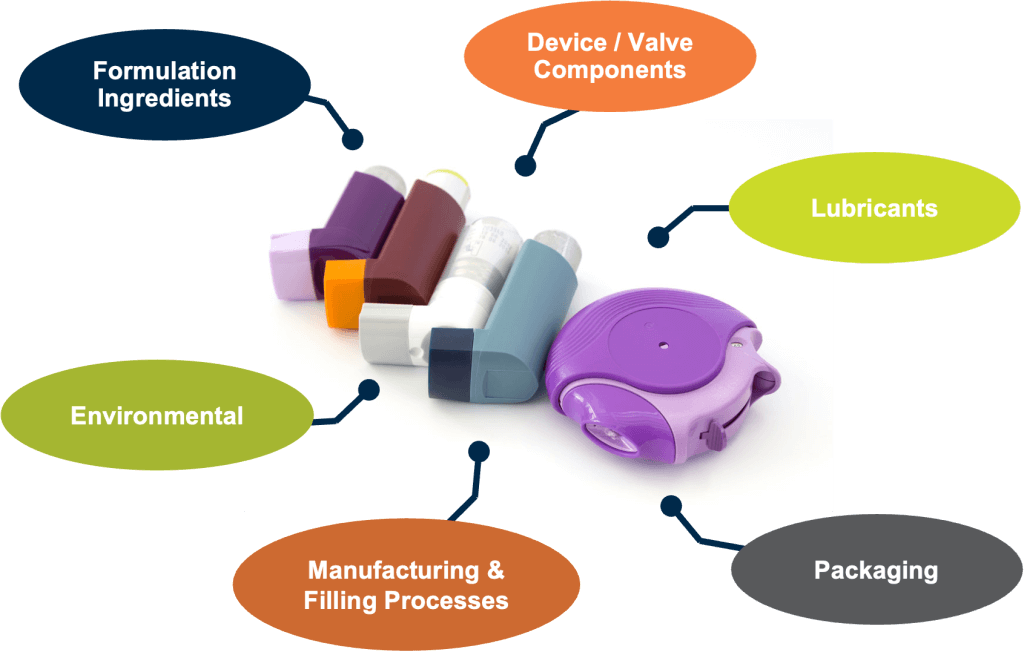
Foreign particles may arise from multiple sources:
- Device components or lubricants
- Formulation ingredients or excipients
- Manufacturing and filling processes
- Environmental or packaging materials
Even at trace levels, these particulates can influence aerosol behavior and performance, stability, and ultimately, patient exposure. Robust characterization provides a clear understanding of what is present and where it originated from — enabling data-driven control strategies and quality assurance.
Regulatory Expectations and Industry Standards
Building on the need for robust characterization, current industry practice calls for a scientifically grounded, risk-based approach to managing foreign particulate matter in inhalable drug products.
During development, full characterization – including identification, source attribution, size, shape, and consistency – is essential to establish control strategies and understand contamination risks. Once product stability is demonstrated, routine quality control typically focuses on enumeration, unless investigating deviations, addressing complaints, or observing unexpected trends in stability.
Increasingly, manufacturers are expected to implement fit-for-purpose testing strategies that include qualitative and quantitative assessments of particulate matter across relevant size ranges.
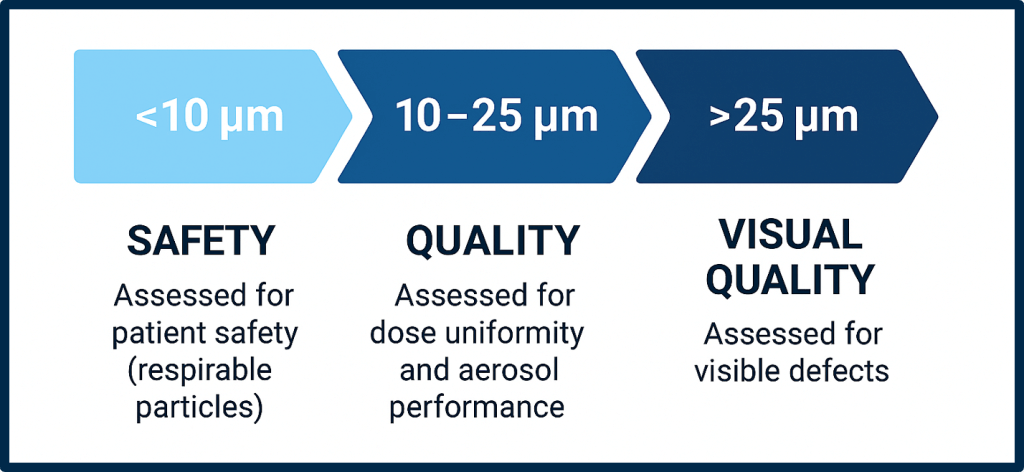
Particle size ranges commonly addressed include 2–10 µm, 10–25 µm, and >25 µm, with smaller particles often evaluated for safety and larger ones for visual or quality concerns.
Regulatory Expectations
Foreign particulate matter is recognized as a Quality Critical Attribute (QCA) for inhalation products such as MDIs and DPIs. Regulatory authorities require a robust control strategy throughout development and lifecycle, including:
- Initial characterization during product development
- Assessment of stability batches
- Testing at batch release, when justified
Key guidelines and standards:
- ICH Q10
Pharmaceutical Quality System: Lifecycle approach to quality and risk management - ICH Q4B Annex 3
Harmonized pharmacopoeial methods for particulate contamination (USP <788>, Ph. Eur. 2.9.19, JP 6.07) - FDA Guidance:
- Metered Dose Inhaler (MDI) and Dry Powder Inhaler (DPI) Drug Products—Quality Considerations
- Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products—CMC Documentation
Sampling methods must be tailored to the specific dosage form and manufacturing process, ensuring that particles are neither introduced nor masked during collection.
This proactive approach not only aligns with evolving regulatory expectations but also strengthens product integrity, patient safety, and inspection readiness.
These principles together define current expectations: a scientifically justified, risk-based strategy for detecting, classifying, and controlling particulate matter throughout product development and lifecycle.
Analytical and Practical Challenges
Despite these clear expectations, the analytical process is far from straightforward. Foreign particles are often sparse, chemically diverse, and embedded in complex formulations. Effective characterization demands careful method design to:
- Preserve particle integrity during formulation removal
- Recover representative samples from pressurized or intricate devices
- Distinguish between intrinsic and extrinsic sources
- Avoid environmental contamination during low-count analyses
- Enable meaningful assessment across a broad size spectrum
Without appropriate techniques, results can be inconsistent or misleading – complicating investigations and regulatory reviews.
A proactive, fit-for-purpose approach to FPM characterization not only meets industry expectations but also reinforces product quality, patient safety, and regulatory confidence.
Our Approach to Foreign Particle Characterization
At Resolian, we apply a fit-for-purpose analytical framework to identify and understand foreign particulate matter in inhalable drug products. Our approach balances sensitivity, traceability, and practicality, enabling manufacturers to trace contamination sources and demonstrate control over their processes.
Beyond routine characterization, we specialize in isolating and identifying single-entity foreign matter – those rare, unexpected contaminants that can trigger investigations or regulatory scrutiny.
This capability ensures rapid root-cause analysis and targeted corrective actions.

Controlled Sampling in Particle-Free Environments
- Custom-built sampling apparatus tailored for pMDIs and other inhalation formats
- Operation within laminar flow cabinets to minimize environmental contamination
- Device actuation into formulation-specific solvents that dissolve active/excipient matrices without compromising foreign particle integrity
- Capture of particles on bespoke filters
- HIAC (light obscuration) analysis to verify particle counts and assess filter load, supporting method development and characterization accuracy
High-Resolution Particle Speciation
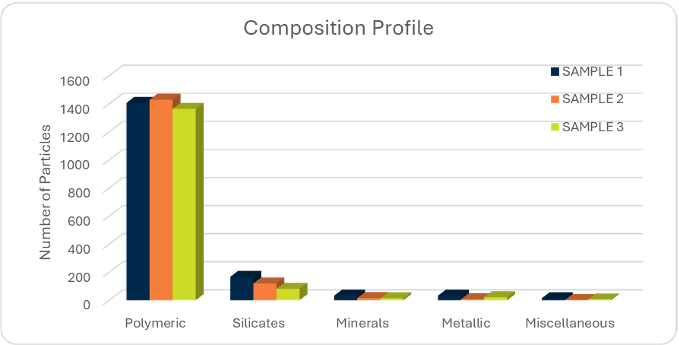
- Elemental profiling using SEM-EDX, analyzing each particle individually
- Categorization by size, composition and likely origin, e.g. mineral, polymeric, metallic, or environmental
- Classification by regulatory-relevant size brackets:
2-5 µm, 5-10 µm, 10–25 µm, >25 µm
This workflow provides both qualitative and quantitative insights, supporting investigations, stability studies, and regulatory submissions with clear, actionable data.
Let’s Collaborate
Foreign particulate characterization isn’t just a regulatory expectation – it’s a cornerstone of quality assurance and risk management in inhalable drug development. Whether you’re troubleshooting a deviation, conducting stability studies, or preparing for regulatory submission, understanding the origin and nature of particulate matter empowers confident, defensible decision-making.
At Resolian, we work closely with manufacturers to:
- Design analytical strategies tailored to your product and process
- Deliver clear, traceable, scientifically-backed data for confident decision-making
- Provide insights that strengthen quality systems and inspection readiness
With our dedicated team, state-of-the-art instrumentation, and custom-engineered sampling solutions, we help you meet the highest standards of compliance and patient safety.
Let’s collaborate.
Contact our analytical sciences team to discuss your needs.


